The Quild Future Unicorn is a weekly product-focused note highlighting early-stage startups with statistically significant signals of becoming unicorns.
Unlearn creates digital twins of patients to enable biopharma partners to run faster, more successful clinical trials. Unlike a traditional clinical trial, a digital twin is created for every patient using a machine learning model trained on historical data.
Founders: Charles Fisher (CEO), Aaron Smith (Head of Machine Learning), and Jonathan Walsh (Head of Data Science)
Signals:
Venture-backed experience
Charles worked as a machine learning engineer at Leap Motion (<1 yr)
Aaron worked as an algorithm engineer at Leap Motion (3+ yrs)
Jonathan Walsh was a data scientist at Leap Motion (1+ yrs)
Fast team growth
Grew 85% YoY to ~70 employees
Top investors
Insight Partners led series B
8VC led series A
Top company alumni
Charles was a scientist at Pfizer (1 yr)
Top university alumni
Charles graduated from University of Michigan (BS) and Harvard University (PhD)
Aaron graduated from Penn State (BS, MS) and University of Pennsylvania (PhD)
Jonathan graduated from University of Chicago (BS) and University of Washington (PhD)
This series is powered by Specter, a data intelligence provider for the world's leading investors like Accel and Bessemer. I have been working with data-driven tools for venture for a long time, and Specter's is the best one.
Product Notes
Problem and persona
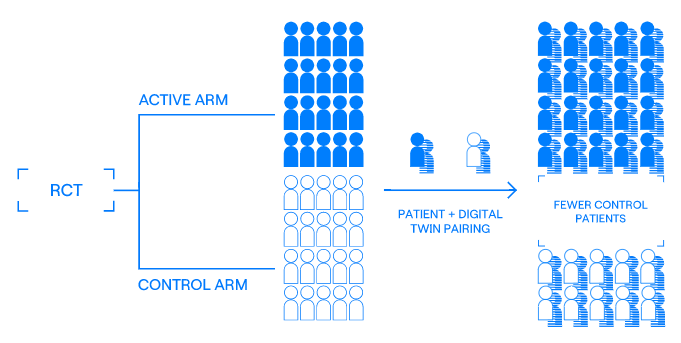
Clinical trials play a crucial role in the advancement of new medical treatments and therapies. To evaluate the safety and effectiveness of these new treatments, randomized controlled trials (RCTs) are widely accepted as the standard method.
Placebo vs active: In RCTs, a placebo is a treatment or substance that has no active therapeutic effect, but is given to one group of participants (the "placebo group") in order to compare the results to another group of participants who receive the active treatment (the "active group"). The use of a placebo allows researchers to determine whether the active treatment is having a specific effect on the participants, or if any changes seen are due to other factors, such as the natural course of the disease or a psychological response to receiving treatment.
Sponsor vs investigator: In clinical trials, the sponsor and the investigator have distinct roles. The sponsor, typically a pharmaceutical or biotech company or a government agency funds and manages the trial, develops the protocol, obtains funding, and ensures compliance with regulations and ethical guidelines. They also handle the trial's overall management, data analysis, and final report. On the other hand, the investigator, a healthcare professional, conducts the trial at a specific site such as a hospital, clinic or research institution, and is responsible for participant recruitment, obtaining informed consent, and ensuring the trial is conducted according to protocol and regulations.
A problem that RCTs face: However, these trials almost always face the challenge of participant recruitment and retention. This delays the study's timeline which takes between 10-15 years and costing up to $2 billion for a single treatment. These difficulties in recruitment can stem from a lack of patient engagement or difficulty in identifying eligible participants. Delays in the study can not only result in financial losses for the sponsors but also damage the reputation of the investigators.
Product
Unlearn generates Digital Twins, which are synthetic humans that can replace control participants. Investigators can reduce the number of human patients required to run the study, accelerating the timeline.
The hurdle that Unlearn overcame is generating digital synthetic humans reliable enough for medical scrutiny. Their model starts with historical clinical trial data, which they carefully curate from multiple sources. A clinical trial dataset is a panel data composed of patients’ medical data over a long period of time. For example, in Unlearn’s initial landmark study they had data 44 variables for ~1,900 patient data over 18-months in 3-month intervals. Every 3 months, each patient underwent multiple tests to measure variables from cognitive ability to cholesterol count. The Unlearn team then used a generative AI model (a conditional restricted Boltzmann machine) to simulate the patients’ variables over time. They found that the synthetic data is hardly distinguishable from the real data.
Unlearn productized the study process as a service to pharmaceutical and biotech companies by following the steps:
Curate datasets
Build a digital twin model
Compare synthetic data to actual trial data